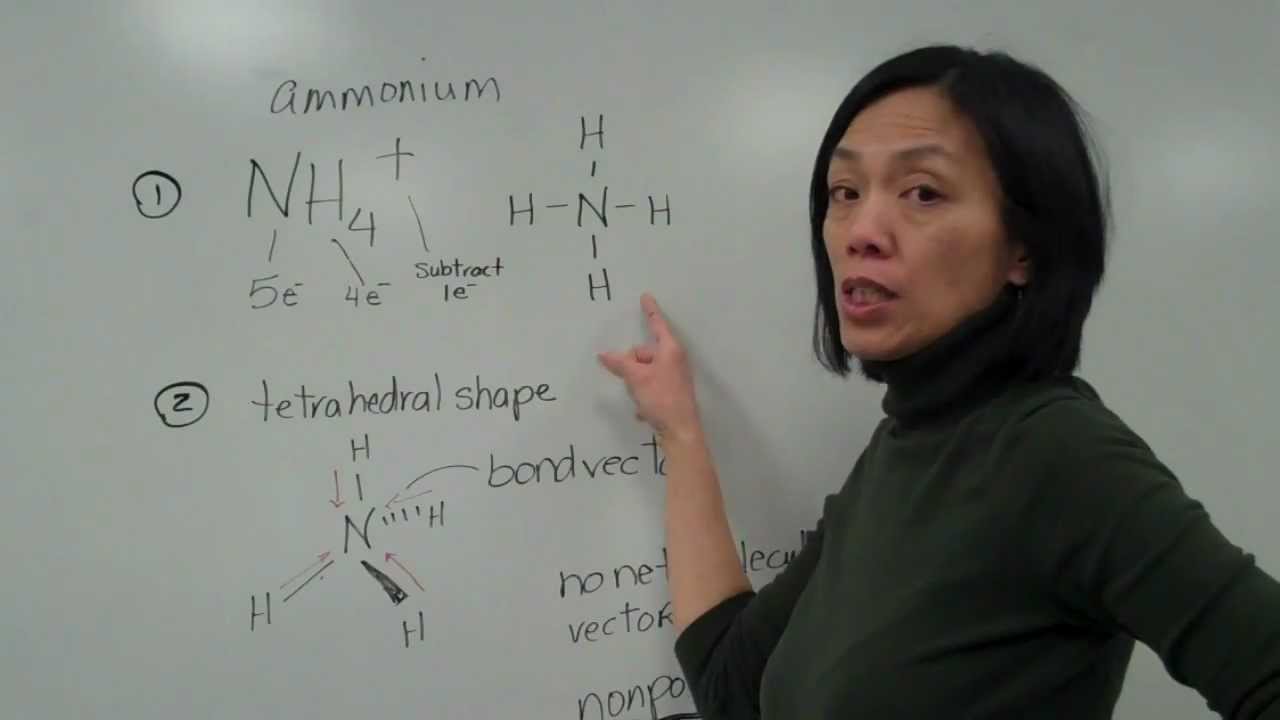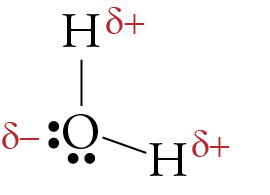Brilliant Strategies Of Tips About How To Find Out If A Molecule Is Polar

Every sufficiently asymmetric molecule will be polar, but some more than others.
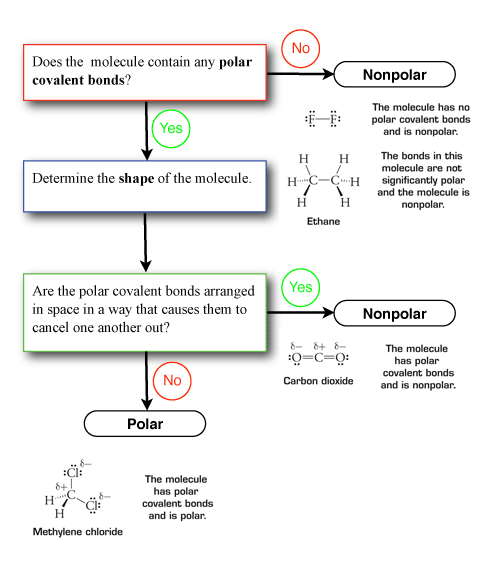
How to find out if a molecule is polar. A substance that contains polar covalent bonds may not be overall polar. This is due to the shape of the molecule. Draw the lewis structure, step 2:
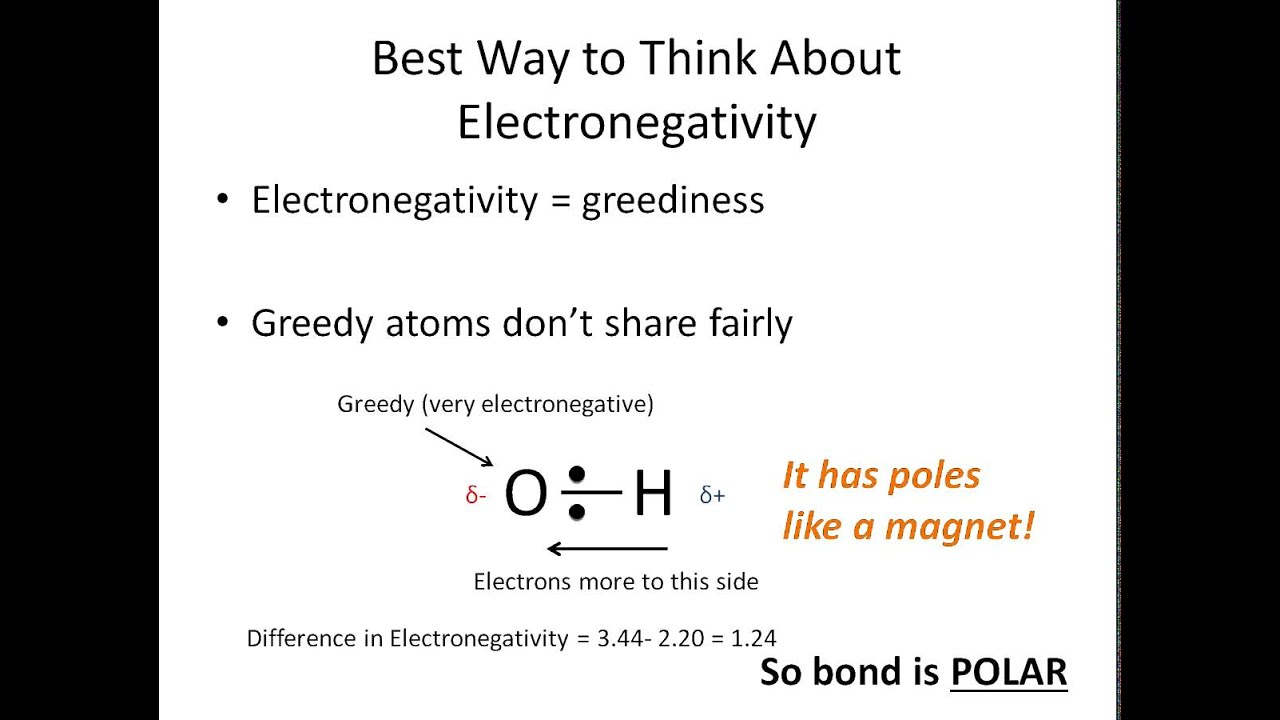
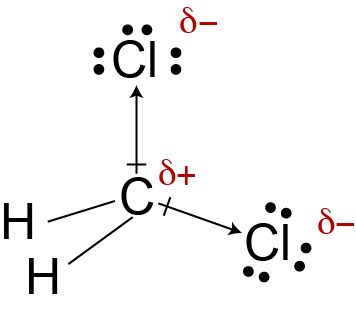
If the difference is 0.4 or larger, the bond will be polar. Atoms at the top right of the table, such as chlorine and oxygen, will. Two things are needed for a molecule to be polar 1) it needs to have polar bonds and 2) there must be asymmetry in the way the bonds are arranged around the atom.
This question can be easily answered by looking at the t. The polarity of molecules is related to the polarity of bonds within the molecule, but just having. Therefore, for the molecule in question, you can tell if it is polar by the structure.
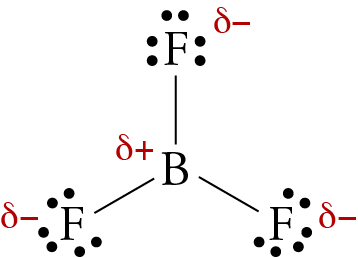
How to determine if a molecule is polar or not? It provides examples so you can quickly distinguish nonpolar molecul. Since the f atoms are in the same plane, their attraction of electrons is that much stronger.
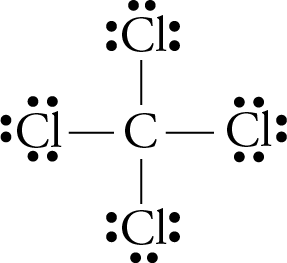
This question can be easily answered by looking at the atoms and lone pair surrounding the central. If the arrangement is symmetrical and the arrows are of equal length, the molecule is nonpolar. By looking at the periodic table, you can tell a lot about how polar a bond will be between any two atoms.
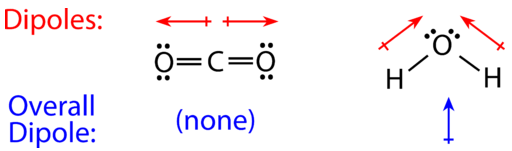
Both of the bonds inside the molecule. A polar molecule has a net dipole as a result of the opposing charges (i.e. Click on the molecule's name.